Our Pipeline
TRACK-NK™ and Coalescent™
Cell Clinical Pipeline
CytoImmune Therapeutics is a clinical-stage biotechnology company, developing a novel class of cancer immunotherapy medicines based on engineered, allogeneic, tumor-reactive natural killer (TRACK-NK™) cell therapies for patients with cancer. The cells are engineered to both directly attack cancer cells and broadly stimulate both the innate and adaptive arms of the immune system through potent IL-15 secretion. This dual mechanism of action of the TRACK-NK™ cells is achieved through proprietary manufacturing methods and engineering the expression of secreted IL-15, chimeric antigen receptors (CAR), and the secretion of a bispecific killer engager (BiKE) molecule, enabling both dual antigen targeting and broad immune stimulation in one engineered cell. The company is headquartered in Duarte, CA with a manufacturing facility for cells and virus in Puerto Rico.
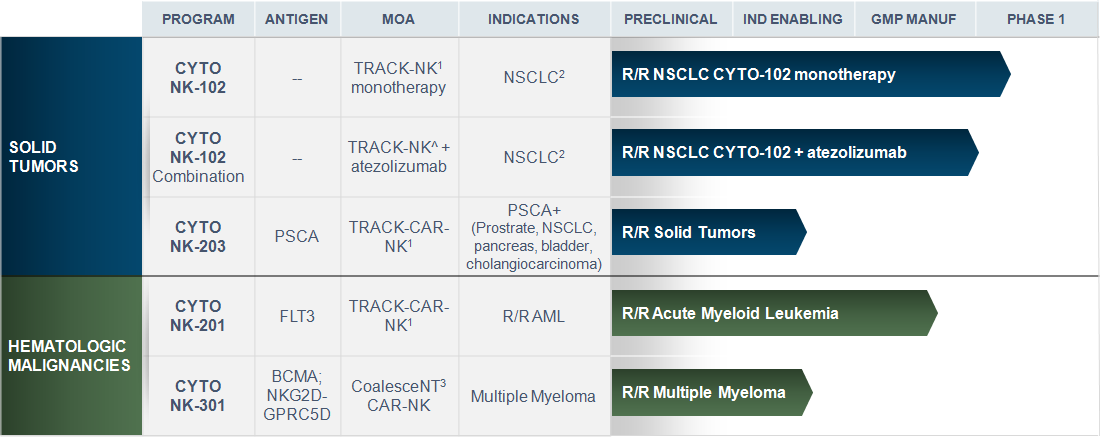
- TRACK-NK Platform: Tumor-Reactive and Anti-PD-L1 Co-stimulated Killer Cells | Tumor targeting is enhanced with triple cytokine induction, and (where indicated) in vivo combination with anti-PD-L1 and CAR expression
- NSCLC is the proof of concept (POC) indication for Cyto-102 with additional indications planned post-POC in NSCLC (e.g. HNSCC)
- CoalesceNT NK Platform: Tumor-reactive NK Cells engineered with triple edits: (1) expression of a CAR (2) secretion of soluble IL-15 and (3) secretion of a BiKE (Bispecific Killer Engager)